Friday, October 30, 2020
Thursday, October 29, 2020
Population-based payments to replace fee-for-service
The rapid decline of in-person visitation in early 2020 due to the COVID-19 pandemic revealed the unstable reality of fee-for-service payments, which should be replaced by population-based payments, authors of an article recently published in JAMA suggest.
Suhas Gondi, BA, and Dave A. Chokshi, MD, MSc, suggest that financial clearance should be a new goal for payment reform. Though CMS designed and implemented various value-based are programs through alternative payment models to simultaneously improve the quality of care and reduce costs, the authors say these efforts have only had modest effects on health outcomes and spending.
Furthermore, the authors write that the pandemic’s effect on in-person visitation reveals that fee-for-service payments are “exceptionally vulnerable to shocks that reduce demand for in-person care.”
On the contrary, the authors suggest that population-based payments are more resilient in the face of shocks like COVID-19 and will protect access to care when it is most needed.
To achieve healthcare population-based payment stability, the authors suggest looking to fully capitated payments.
In a paper published in 2016 in Pediatrics, Steven A. Farmer et. al. calculated the hypothetical break-even point for a pediatric practice and provide sample income statement calculations. Overall, though written with a pediatric practice in mind, capitation income can be calculated by:
Net Income = Patient Co-payments + Capitation Base Rate + Utilization Incentives + Quality − Operating Expenses.
The authors of the JAMA paper also suggest looking to the ACO Investment Model of CMS and Hawaii’s experience with population-based payments for primary care. Other emerging examples offered include the Blue Cross Blue Shield of North Carolina Accelerate to Value program or the Blue Cross Blue Shield of Massachusetts pilot for independent primary care practice, both of which the authors suggest should speed implementation.
"By abandoning the fee-for-service reimbursements that disproportionately reward specialist and procedural care, true population-based payments at the organizational level may stimulate redistribution of resources from specialists toward primary care, improving population health,” the authors write.
 Manual Prescription Pad (Large - Yellow)
Manual Prescription Pad (Large - Yellow)
 Manual Prescription Pad (Large - Pink)
Manual Prescription Pad (Large - Pink)
 Manual Prescription Pads (Bright Orange)
Manual Prescription Pads (Bright Orange)
 Manual Prescription Pads (Light Pink)
Manual Prescription Pads (Light Pink)
 Manual Prescription Pads (Light Yellow)
Manual Prescription Pads (Light Yellow)
 Manual Prescription Pad (Large - Blue)
Manual Prescription Pad (Large - Blue)
Suhas Gondi, BA, and Dave A. Chokshi, MD, MSc, suggest that financial clearance should be a new goal for payment reform. Though CMS designed and implemented various value-based are programs through alternative payment models to simultaneously improve the quality of care and reduce costs, the authors say these efforts have only had modest effects on health outcomes and spending.
Furthermore, the authors write that the pandemic’s effect on in-person visitation reveals that fee-for-service payments are “exceptionally vulnerable to shocks that reduce demand for in-person care.”
On the contrary, the authors suggest that population-based payments are more resilient in the face of shocks like COVID-19 and will protect access to care when it is most needed.
To achieve healthcare population-based payment stability, the authors suggest looking to fully capitated payments.
In a paper published in 2016 in Pediatrics, Steven A. Farmer et. al. calculated the hypothetical break-even point for a pediatric practice and provide sample income statement calculations. Overall, though written with a pediatric practice in mind, capitation income can be calculated by:
Net Income = Patient Co-payments + Capitation Base Rate + Utilization Incentives + Quality − Operating Expenses.
The authors of the JAMA paper also suggest looking to the ACO Investment Model of CMS and Hawaii’s experience with population-based payments for primary care. Other emerging examples offered include the Blue Cross Blue Shield of North Carolina Accelerate to Value program or the Blue Cross Blue Shield of Massachusetts pilot for independent primary care practice, both of which the authors suggest should speed implementation.
"By abandoning the fee-for-service reimbursements that disproportionately reward specialist and procedural care, true population-based payments at the organizational level may stimulate redistribution of resources from specialists toward primary care, improving population health,” the authors write.
Medical Practice Supplies
VIEW ALL
 Manual Prescription Pad (Large - Yellow)
Manual Prescription Pad (Large - Yellow) Manual Prescription Pad (Large - Pink)
Manual Prescription Pad (Large - Pink) Manual Prescription Pads (Bright Orange)
Manual Prescription Pads (Bright Orange) Manual Prescription Pads (Light Pink)
Manual Prescription Pads (Light Pink) Manual Prescription Pads (Light Yellow)
Manual Prescription Pads (Light Yellow) Manual Prescription Pad (Large - Blue)
Manual Prescription Pad (Large - Blue)__________________________________________________
Appointment Reminder Cards
$44.05
15% Off
$56.30
15% Off
$44.05
15% Off
$44.05
15% Off
$56.30
15% Off
VIEW ALL PRODUCTS
Wednesday, October 28, 2020
Election risk management for medical practices
November third brings us the most contentious election in many voter’s lifetimes. These are some of the non-medical risks medical practice managers and owners should consider today.
Asset protection and risk management for medical practice owners is becoming increasingly broad in scope due to both internal and external risks. The 2020 election, combined with the COVID-19 pandemic, adds layers to his complexity in areas ranging from physical security to employment law. We have covered some of these issues before in significant detail, although perhaps not in this specific context, and provide a checklist of specific issues and risks to evaluate now.
City location: Some risks may be completely incidental to your practice but may affect you (and your family) based on issues ranging from your physical proximity to sites of potential conflict like a polling place, hospital, government building, or any other site that may be considered to be politically charged. This risk can happen anywhere, but obviously varies from city to city, so take local factors into account. Consider seeking guidance on security issues, traffic and road closures, and any recommended precautions or warnings from local authorities, law enforcement, and others that may affect the operation of your business (including simple street access) or the safety of your staff and patients.
State, City: As jarring as it is to have to say this about the U.S. for first time in most of our lifetimes, at least ten states are at elevated risk for militia-related political violence before, on, and after election day. According to one of the many reports I’ve reviewed on this issue recently, there are ten states where authorities are on high alert. Practice leaders in Georgia, Michigan, Pennsylvania, Wisconsin, and Oregon should be on high alert. The report also states that North Carolina, Texas, Virginia, California, and New Mexico are at an elevated riskof violence and other disruptions.
A wide variety of outlandish disinformation and conspiracy theories are being promoted across social media. Unfortunately, some of it specifically targets medical professionals, hospitals, and the healthcare industry, creating a physical security risk for both people and facilities. While all medical professionals should exercise caution and increased situational awareness, those with high profiles in their profession, those who are frontline caregivers, those who are easily identifiable as doctors or nurses (scrubs, ID cards, etc. outside a practice setting) and those visible and vocal on social media should be extra careful.
Just a few of the many fantastic tales that could make providers a target include:
 Manual Prescription Pad (Large - Yellow)
Manual Prescription Pad (Large - Yellow)
 Manual Prescription Pad (Large - Pink)
Manual Prescription Pad (Large - Pink)
 Manual Prescription Pads (Bright Orange)
Manual Prescription Pads (Bright Orange)
 Manual Prescription Pads (Light Pink)
Manual Prescription Pads (Light Pink)
 Manual Prescription Pads (Light Yellow)
Manual Prescription Pads (Light Yellow)
 Manual Prescription Pad (Large - Blue)
Manual Prescription Pad (Large - Blue)
Asset protection and risk management for medical practice owners is becoming increasingly broad in scope due to both internal and external risks. The 2020 election, combined with the COVID-19 pandemic, adds layers to his complexity in areas ranging from physical security to employment law. We have covered some of these issues before in significant detail, although perhaps not in this specific context, and provide a checklist of specific issues and risks to evaluate now.
Incidental Physical Risk: Geography
City location: Some risks may be completely incidental to your practice but may affect you (and your family) based on issues ranging from your physical proximity to sites of potential conflict like a polling place, hospital, government building, or any other site that may be considered to be politically charged. This risk can happen anywhere, but obviously varies from city to city, so take local factors into account. Consider seeking guidance on security issues, traffic and road closures, and any recommended precautions or warnings from local authorities, law enforcement, and others that may affect the operation of your business (including simple street access) or the safety of your staff and patients.
State, City: As jarring as it is to have to say this about the U.S. for first time in most of our lifetimes, at least ten states are at elevated risk for militia-related political violence before, on, and after election day. According to one of the many reports I’ve reviewed on this issue recently, there are ten states where authorities are on high alert. Practice leaders in Georgia, Michigan, Pennsylvania, Wisconsin, and Oregon should be on high alert. The report also states that North Carolina, Texas, Virginia, California, and New Mexico are at an elevated riskof violence and other disruptions.
Conspiracy Theories Directly Targeting Healthcare Professionals
A wide variety of outlandish disinformation and conspiracy theories are being promoted across social media. Unfortunately, some of it specifically targets medical professionals, hospitals, and the healthcare industry, creating a physical security risk for both people and facilities. While all medical professionals should exercise caution and increased situational awareness, those with high profiles in their profession, those who are frontline caregivers, those who are easily identifiable as doctors or nurses (scrubs, ID cards, etc. outside a practice setting) and those visible and vocal on social media should be extra careful.
Just a few of the many fantastic tales that could make providers a target include:
- Medical professionals are falsely inflating COVID19 cases and death reporting for a huge payday and political purposes. “There are no full hospitals or ER rooms”.
- COVID-19 is a complete hoax that is being perpetrated on America to derail the economy and one candidate with the help of the medical industry
- The virus is real, but some doctors are hiding the real cures and treatments and silencing the doctorssharing miracle cures that people should be using
Other Issues to Consider In the Context of the Election
- Consider the physical security of your medical practice and any short term on long term changes you need to make
- Examine the adequacy (or existence) of your firearms related risk management policies regarding both patient and third-party possession of firearms in your facility and your internal firearms policy for staff
- Review your business loss and liability insurance coverage in all areas including your potential need for specialized coverage for acts of violence
- Understand the legal regulation of any last minute campaign contributions, and those involving your practice in particular
- Be prepared to manage additional, politically motivated aggression and frivolous arguments to be made by those that may be triggered by your office safety, screening and mask policies. Train staff to reduce conflict by having and enforcing an effective mask policy and procedures
- Manage employment law risks carefully, especially for the next 30 days. Lead by example, have a plan and rules in place to manage any conflict between employees, and do not tie any aspect of an employment relationship to any political issue or allow harassment or discrimination of staff members by each other. Finally, keep an eye out for potentially inappropriate political displays and memorabilia, speech and apparel including Halloween costumes.
Medical Practice Supplies
VIEW ALL
 Manual Prescription Pad (Large - Yellow)
Manual Prescription Pad (Large - Yellow) Manual Prescription Pad (Large - Pink)
Manual Prescription Pad (Large - Pink) Manual Prescription Pads (Bright Orange)
Manual Prescription Pads (Bright Orange) Manual Prescription Pads (Light Pink)
Manual Prescription Pads (Light Pink) Manual Prescription Pads (Light Yellow)
Manual Prescription Pads (Light Yellow) Manual Prescription Pad (Large - Blue)
Manual Prescription Pad (Large - Blue)__________________________________________________
Appointment Reminder Cards
$44.05
15% Off
$56.30
15% Off
$44.05
15% Off
$44.05
15% Off
$56.30
15% Off
VIEW ALL PRODUCTS
Tuesday, October 27, 2020
Managing BRCA results from 23andMe
During a routine office visit, a patient mentions that she recently submitted a saliva sample to 23andMe. The company performed BRCA analysis and the patient wants to discuss the results with you. How should you approach the discussion?

A generation ago, the Internet gave patients unprecedented access to medical information and thereby changed the nature of doctor-patient interaction. Rather than physicians being the sole source of medical information, patients began accessing information online and then bringing it to their physicians for discussion.
The approval of 23andMe’s BRCA test changed the roles of doctor and patient yet again, as patients are now ordering genetic tests themselves and bringing the results to their physicians for interpretation. Whether this moves the doctor-patient relationship in the right direction is debatable; however, there is no debate that these test results can be clinically significant and must be handled appropriately. This article provides a foundation for doing so.
“Consumer” genetics, also known as “over-the-counter” genetics, is initiated by patients without the authorization of a clinician. It began in 2006 as an entertaining way of tracing family lineage and quickly became very popular, with more than 5 million people having submitted samples for analysis.1
In 2015, the focus expanded from recreational into clinical when 23andMe began testing for Bloom Syndrome, a rare autosomal recessive disorder of gene BLM. BRCA analysis was added in 2018, and the 23andMe panel now includes 13 genetic diseases.2 The company also offers carrier testing for autosomal recessive conditions (e.g., cystic fibrosis), which it markets to couples who are planning to have children.
Although 23andMe provides consumers with personalized information on numerous medical conditions, it does not provide one-on-one clinical interaction whereby this type of information is normally shared. Instead, consumers are notified when their results become available, and they can directly access the information online, where it is summarized in a “Genetic Health Risk Report.”
To prepare consumers for the possibility of “bad news”, they are required to watch an educational video prior to accessing any result that 23andMe deems “potentially sensitive” (e.g., BRCA, Parkinson’s disease).
The absence of a one-on-one relationship with a healthcare provider is a serious shortcoming, especially for patients who learn that they harbor a pathogenic mutation by reading a report on their mobile device. They are often emotionally devastated and frequently turn to their physicians for guidance.
Both the Genetic Health Risk Report and the educational video are of good quality, with the report having a demonstrated user comprehension rate of greater than 90% [J1]and the video having been created by certified genetic counselors.3
However, the absence of a one-on-one relationship with a healthcare provider is a serious shortcoming, especially for patients who learn that they harbor a pathogenic mutation by reading a report on their mobile device.4 They are often emotionally devastated and frequently turn to their physicians for guidance.
In terms of responding to these patients, it is important to first understand the nature of the test. Although 23andMe’s methodology has high levels of both accuracy (> 99% concordance with Sanger sequencing (which has been the gold standard for genetic sequencing for 40 years) and reproducibility (> 99%), the test has numerous limitations.5
First, because the saliva samples are collected in a non-clinical setting, specimen integrity could be compromised. This risk is elevated by the common practice of multiple friends or family members collecting, labelling, and submitting their specimens in the same setting.
Second, in contrast to high-end, clinical-grade labs, which always confirm pathogenic findings with Sanger sequencing before issuing a report, 23andMe’s reports are based on genotyping alone, without any confirmation. Finally, although there are more than 1000 pathogenic BRCA mutations, 23andMe tests for only the three mutations that are common in the Ashkenazi Jewish population (BRCA1 185delAG, BRCA 15382insC, and BRCA2 6174delT).6
However, even among Ashkenazi Jews, this approach has limitations because they can occasionally have non-Ashkenazi mutations.7 So, given the numerous limitations, the US Food and Drug Administration requires 23andMe to include the following warning in its Genetic Health Risk Reports: This test is not a substitute for visits to a healthcare provider for recommended screenings…and should not be used to determine any treatments.
In returning to our hypothetical patient who wants to discuss her 23andMe results, there are several possible scenarios:
Unless the patient made a mistake in collecting and labelling her specimen, the likelihood of her, indeed, having a pathogenic mutation is greater than 99%. However, because 23andMe’s methodology has numerous limitations and its reports contain a disclaimer, no treatment decisions should be made until the result is confirmed with a sample collected under medical supervision and processed by a high-end, clinical-grade lab.
To expedite this process, the lab should be informed of the mutation that was identified and asked to confirm its presence with single-site testing. This approach is both faster and less expensive than full panel testing. In most cases, the pathogenic mutation will be confirmed and the patient can be managed accordingly.
However, on rare occasions, the pathogenic mutation will not be confirmed, and the clinician will thus be left with conflicting results: a positive from 23andMe and a negative from a clinical-grade lab. This scenario should always be resolved in favor of the clinical-grade lab.
Those labs not only have higher levels of accuracy, when performing single-site testing, they always confirm both positives and negatives with Sanger sequencing. A negative result on single-site testing from a high-end lab would thus represent two negative results (one by NextGen sequencing and one by Sanger sequencing). Because this methodology is vastly superior to the genotyping performed by 23andMe, conflicting results should always be resolved in favor of the clinical-grade lab.
While this is certainly better than a positive result, it means only that the patient most likely does not have one of the three Ashkenazi founder mutations. However, there are more than 1000 other pathogenic mutations that she could have.
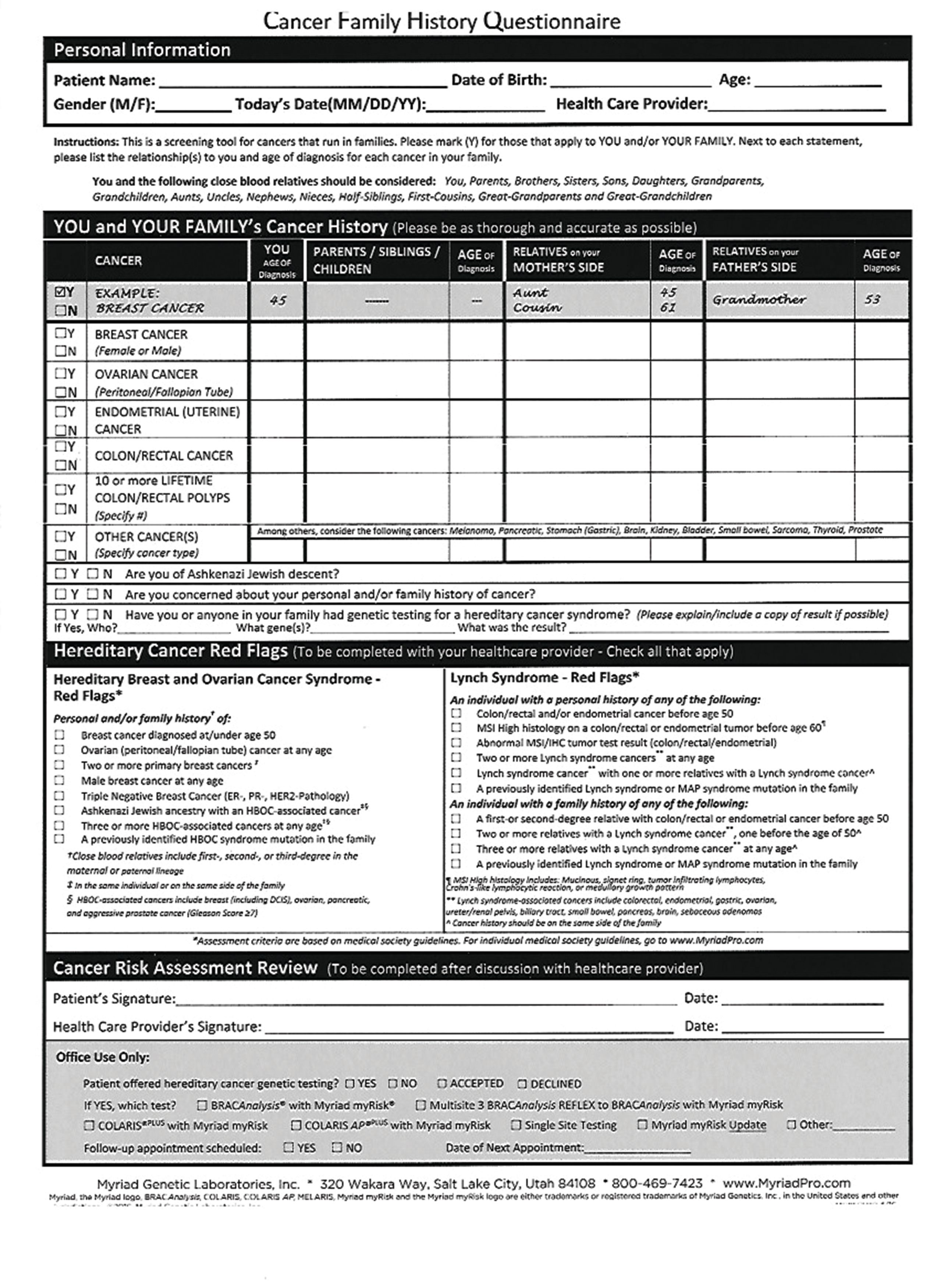
In terms of responding to this patient, you should first explain the limitations of the 23andMe test and then use the discussion as an opportunity to determine whether she meets criteria for clinical-grade testing (Table 1).8,9
Between 5% and 15% of women meet criteria, 1 in 400 harbor a pathogenic mutation, and many others will be identified as being high-risk negative (i.e., they do not have a pathogenic mutation but are nonetheless at very high risk of developing cancer.) The patient’s request to discuss her 23andMe result could thus serve as a gateway to clinical-grade testing and, with it, significant changes in her management.
As in Scenario 2, your response to this patient should begin by updating her personal and family history to determine if she meets criteria for clinical-grade testing.
Ideally, practitioners can incorporate this “cancer” family history questionnaire (Table 1) into their existing electronic medical record system. If this results in the patient having such testing, then there is nothing to be gained by also testing with 23andMe. Positive results can be interpreted by the practitioner and/or in conjunction with a clinical genetic counselor.
On the other hand, if the patient does not meet criteria for clinical-grade testing (and thus does not qualify for insurance coverage), then the role of the 23andMe test is not as clear. On one hand, testing patients who do not meet formal criteria is usually discouraged because it wastes resources and exposes them to the risks of a false positive.
However, in this situation, the patient is spending her own money and the risk of a false positive is less than 1%. In addition, even if she suffers a false positive, the potential harm consists only of having to perform a second saliva (or blood) test and some temporary anxiety. The traditional reasons for discouraging testing are thus far from compelling.
There is also a growing concern that testing only in accordance with our current criteria may be missing almost 50% of patients who harbor pathogenic mutations. A study of patients with breast cancer found that incidence of pathogenic mutations was 8.7% in the 477 patients who met criteria and 7.9% in the 479 patients who did not meet criteria.10
Similar results have been reported by other authors11 and also by 23andMe, which states, on it’s website, that 50% of pathogenic mutations have been found in patients who did not have a first-degree relative with breast or ovarian cancer (though the formal criteria include other cancers as well as second degree relatives).
With these added studies, a good argument can be made that testing should not be strictly limited to patients who meet criteria. In fact, the American Society of Breast Surgeons recently adopted just such a position and now recommends that all women diagnosed with breast cancer undergo germline genetic testing.12
So, how should you respond when a patient who does not meet criteria for clinical-grade testing asks whether she should have BRCA testing by 23andMe?
Although 23andMe’s methodology detects only three mutations, they are among the most common mutations and the test is highly accurate in identifying them.
Given the somewhat poor sensitivity of the criteria that we use for clinical-grade testing, the relatively low cost of 23andMe’s test ($50-$100), and the fact that the patient would be spending her own money, there is little reason to dissuade her from having the test if she understands its limitations and the significance of the results.
Although consumer-initiated genetic testing should not be used to guide treatment, it can be helpful in identifying patients who harbor pathogenic BRCA mutations, particularly those who do not meet formal criteria and thus do not qualify for insurance coverage of clinical-grade testing.
The tools provided in these clinical scenarios may help guide practitioners in managing the patient who presents with a 23andMe genetic report.
1. 23andMe Statistics, Facts and History. https://www.reviewchatter.com/statistics-facts-history/23andMe/ Accessed November 13, 2018.
2. 23andMe. Reports included in all services. https://www.23andme.com/dna-reports-list/ Accessed January 10, 2020.
3. FAQs-23andMe for Medical Professionals. https.//www.Medical.23andMe.com-FAQ/
4. Pomerantz D. 23andMe had devastating news about my health. I wish a person had delivered it. https://www.statnews.com/2019/08/08/23andme-genetic-test-revealed-high-cancer-risk/ Accessed January 8, 2020.
5. 23andMe. Understanding our scientific process. https://medical.23andme.com/about-our-test/ Accessed January 10, 2020.
6. 23andMe. Do you speak BRCA? https://www.23andme.com/brca/ Accessed January 10, 2020.
7. Kauff ND, Perez-Segura P, Robson ME, Scheuer L, Siegel B, Schluger A, et al. Incidence of non-founder BRCA1 and BRCA2 mutations in high risk Ashkenazi breast and ovarian cancer families. J Med Genetics. https://jmg.bmj.com/content/39/8/611/
8. NCCN Clinical Practice Guidelines in Oncology-Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. https://www.genomeweb.com/sites/default/files/nccn_guidelines_genfamhighrisk_breastovarianpancreatic_v1.2020/
9. What are the NCCN guidelines for colorectal cancer screening in patients with hereditary nonpolyposis colorectal cancer (HNPCC) (Lynch syndrome)? https://www.medscape.com/answers/2500006-10897/what-are-the-nccn-guidelines-for-colorectal-cancer-screening-in-patients-with-hereditary-nonpolyposis-colorectal-cancer-hnpcc-lynch-syndrome./
10. Beitsch PD, Whitworth PW, Hughes K Patel R, Rosen B, Compagnoni G. Underdiagnosis of hereditary breast cancer: Are genetic testing guidelines a tool or an obstacle? J Clin Oncology. 2019;37:453-460.
11. King MC, Levy-Lahad E, Lahad A. Population-based screening for BRCA1 and BRCA2. JAMA. 2014;312:1091-1092.
12. American Society of Breast Surgeons. Consensus guideline on genetic testing for hereditary breast cancer. https://www.breastsurgeons.org/docs/statements/Consensus-Guideline-on-Genetic-Testing-for-Hereditary-Breast-Cancer.pdf
 Manual Prescription Pad (Large - Yellow)
Manual Prescription Pad (Large - Yellow)
 Manual Prescription Pad (Large - Pink)
Manual Prescription Pad (Large - Pink)
 Manual Prescription Pads (Bright Orange)
Manual Prescription Pads (Bright Orange)
 Manual Prescription Pads (Light Pink)
Manual Prescription Pads (Light Pink)
 Manual Prescription Pads (Light Yellow)
Manual Prescription Pads (Light Yellow)
 Manual Prescription Pad (Large - Blue)
Manual Prescription Pad (Large - Blue)

A generation ago, the Internet gave patients unprecedented access to medical information and thereby changed the nature of doctor-patient interaction. Rather than physicians being the sole source of medical information, patients began accessing information online and then bringing it to their physicians for discussion.
The approval of 23andMe’s BRCA test changed the roles of doctor and patient yet again, as patients are now ordering genetic tests themselves and bringing the results to their physicians for interpretation. Whether this moves the doctor-patient relationship in the right direction is debatable; however, there is no debate that these test results can be clinically significant and must be handled appropriately. This article provides a foundation for doing so.
“Consumer” genetics, also known as “over-the-counter” genetics, is initiated by patients without the authorization of a clinician. It began in 2006 as an entertaining way of tracing family lineage and quickly became very popular, with more than 5 million people having submitted samples for analysis.1
In 2015, the focus expanded from recreational into clinical when 23andMe began testing for Bloom Syndrome, a rare autosomal recessive disorder of gene BLM. BRCA analysis was added in 2018, and the 23andMe panel now includes 13 genetic diseases.2 The company also offers carrier testing for autosomal recessive conditions (e.g., cystic fibrosis), which it markets to couples who are planning to have children.
Although 23andMe provides consumers with personalized information on numerous medical conditions, it does not provide one-on-one clinical interaction whereby this type of information is normally shared. Instead, consumers are notified when their results become available, and they can directly access the information online, where it is summarized in a “Genetic Health Risk Report.”
To prepare consumers for the possibility of “bad news”, they are required to watch an educational video prior to accessing any result that 23andMe deems “potentially sensitive” (e.g., BRCA, Parkinson’s disease).
The absence of a one-on-one relationship with a healthcare provider is a serious shortcoming, especially for patients who learn that they harbor a pathogenic mutation by reading a report on their mobile device. They are often emotionally devastated and frequently turn to their physicians for guidance.
Both the Genetic Health Risk Report and the educational video are of good quality, with the report having a demonstrated user comprehension rate of greater than 90% [J1]and the video having been created by certified genetic counselors.3
However, the absence of a one-on-one relationship with a healthcare provider is a serious shortcoming, especially for patients who learn that they harbor a pathogenic mutation by reading a report on their mobile device.4 They are often emotionally devastated and frequently turn to their physicians for guidance.
In terms of responding to these patients, it is important to first understand the nature of the test. Although 23andMe’s methodology has high levels of both accuracy (> 99% concordance with Sanger sequencing (which has been the gold standard for genetic sequencing for 40 years) and reproducibility (> 99%), the test has numerous limitations.5
First, because the saliva samples are collected in a non-clinical setting, specimen integrity could be compromised. This risk is elevated by the common practice of multiple friends or family members collecting, labelling, and submitting their specimens in the same setting.
Second, in contrast to high-end, clinical-grade labs, which always confirm pathogenic findings with Sanger sequencing before issuing a report, 23andMe’s reports are based on genotyping alone, without any confirmation. Finally, although there are more than 1000 pathogenic BRCA mutations, 23andMe tests for only the three mutations that are common in the Ashkenazi Jewish population (BRCA1 185delAG, BRCA 15382insC, and BRCA2 6174delT).6
However, even among Ashkenazi Jews, this approach has limitations because they can occasionally have non-Ashkenazi mutations.7 So, given the numerous limitations, the US Food and Drug Administration requires 23andMe to include the following warning in its Genetic Health Risk Reports: This test is not a substitute for visits to a healthcare provider for recommended screenings…and should not be used to determine any treatments.
In returning to our hypothetical patient who wants to discuss her 23andMe results, there are several possible scenarios:
Scenario 1:The report is positive for a pathogenic BRCA mutation.
Unless the patient made a mistake in collecting and labelling her specimen, the likelihood of her, indeed, having a pathogenic mutation is greater than 99%. However, because 23andMe’s methodology has numerous limitations and its reports contain a disclaimer, no treatment decisions should be made until the result is confirmed with a sample collected under medical supervision and processed by a high-end, clinical-grade lab.
To expedite this process, the lab should be informed of the mutation that was identified and asked to confirm its presence with single-site testing. This approach is both faster and less expensive than full panel testing. In most cases, the pathogenic mutation will be confirmed and the patient can be managed accordingly.
However, on rare occasions, the pathogenic mutation will not be confirmed, and the clinician will thus be left with conflicting results: a positive from 23andMe and a negative from a clinical-grade lab. This scenario should always be resolved in favor of the clinical-grade lab.
Those labs not only have higher levels of accuracy, when performing single-site testing, they always confirm both positives and negatives with Sanger sequencing. A negative result on single-site testing from a high-end lab would thus represent two negative results (one by NextGen sequencing and one by Sanger sequencing). Because this methodology is vastly superior to the genotyping performed by 23andMe, conflicting results should always be resolved in favor of the clinical-grade lab.
Scenario 2:The report is negative for a pathogenic BRCA mutation.
While this is certainly better than a positive result, it means only that the patient most likely does not have one of the three Ashkenazi founder mutations. However, there are more than 1000 other pathogenic mutations that she could have.
In terms of responding to this patient, you should first explain the limitations of the 23andMe test and then use the discussion as an opportunity to determine whether she meets criteria for clinical-grade testing (Table 1).8,9
Between 5% and 15% of women meet criteria, 1 in 400 harbor a pathogenic mutation, and many others will be identified as being high-risk negative (i.e., they do not have a pathogenic mutation but are nonetheless at very high risk of developing cancer.) The patient’s request to discuss her 23andMe result could thus serve as a gateway to clinical-grade testing and, with it, significant changes in her management.
Scenario 3:The patient has not yet submitted her sample to 23andMe. She originally planned to undergo just genealogy testing (to determine her heritage), but asks if she should also have BRCA testing.
As in Scenario 2, your response to this patient should begin by updating her personal and family history to determine if she meets criteria for clinical-grade testing.
Ideally, practitioners can incorporate this “cancer” family history questionnaire (Table 1) into their existing electronic medical record system. If this results in the patient having such testing, then there is nothing to be gained by also testing with 23andMe. Positive results can be interpreted by the practitioner and/or in conjunction with a clinical genetic counselor.
On the other hand, if the patient does not meet criteria for clinical-grade testing (and thus does not qualify for insurance coverage), then the role of the 23andMe test is not as clear. On one hand, testing patients who do not meet formal criteria is usually discouraged because it wastes resources and exposes them to the risks of a false positive.
However, in this situation, the patient is spending her own money and the risk of a false positive is less than 1%. In addition, even if she suffers a false positive, the potential harm consists only of having to perform a second saliva (or blood) test and some temporary anxiety. The traditional reasons for discouraging testing are thus far from compelling.
There is also a growing concern that testing only in accordance with our current criteria may be missing almost 50% of patients who harbor pathogenic mutations. A study of patients with breast cancer found that incidence of pathogenic mutations was 8.7% in the 477 patients who met criteria and 7.9% in the 479 patients who did not meet criteria.10
Similar results have been reported by other authors11 and also by 23andMe, which states, on it’s website, that 50% of pathogenic mutations have been found in patients who did not have a first-degree relative with breast or ovarian cancer (though the formal criteria include other cancers as well as second degree relatives).
With these added studies, a good argument can be made that testing should not be strictly limited to patients who meet criteria. In fact, the American Society of Breast Surgeons recently adopted just such a position and now recommends that all women diagnosed with breast cancer undergo germline genetic testing.12
So, how should you respond when a patient who does not meet criteria for clinical-grade testing asks whether she should have BRCA testing by 23andMe?
Although 23andMe’s methodology detects only three mutations, they are among the most common mutations and the test is highly accurate in identifying them.
Given the somewhat poor sensitivity of the criteria that we use for clinical-grade testing, the relatively low cost of 23andMe’s test ($50-$100), and the fact that the patient would be spending her own money, there is little reason to dissuade her from having the test if she understands its limitations and the significance of the results.
Although consumer-initiated genetic testing should not be used to guide treatment, it can be helpful in identifying patients who harbor pathogenic BRCA mutations, particularly those who do not meet formal criteria and thus do not qualify for insurance coverage of clinical-grade testing.
The tools provided in these clinical scenarios may help guide practitioners in managing the patient who presents with a 23andMe genetic report.
References:
1. 23andMe Statistics, Facts and History. https://www.reviewchatter.com/statistics-facts-history/23andMe/ Accessed November 13, 2018.
2. 23andMe. Reports included in all services. https://www.23andme.com/dna-reports-list/ Accessed January 10, 2020.
3. FAQs-23andMe for Medical Professionals. https.//www.Medical.23andMe.com-FAQ/
4. Pomerantz D. 23andMe had devastating news about my health. I wish a person had delivered it. https://www.statnews.com/2019/08/08/23andme-genetic-test-revealed-high-cancer-risk/ Accessed January 8, 2020.
5. 23andMe. Understanding our scientific process. https://medical.23andme.com/about-our-test/ Accessed January 10, 2020.
6. 23andMe. Do you speak BRCA? https://www.23andme.com/brca/ Accessed January 10, 2020.
7. Kauff ND, Perez-Segura P, Robson ME, Scheuer L, Siegel B, Schluger A, et al. Incidence of non-founder BRCA1 and BRCA2 mutations in high risk Ashkenazi breast and ovarian cancer families. J Med Genetics. https://jmg.bmj.com/content/39/8/611/
8. NCCN Clinical Practice Guidelines in Oncology-Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. https://www.genomeweb.com/sites/default/files/nccn_guidelines_genfamhighrisk_breastovarianpancreatic_v1.2020/
9. What are the NCCN guidelines for colorectal cancer screening in patients with hereditary nonpolyposis colorectal cancer (HNPCC) (Lynch syndrome)? https://www.medscape.com/answers/2500006-10897/what-are-the-nccn-guidelines-for-colorectal-cancer-screening-in-patients-with-hereditary-nonpolyposis-colorectal-cancer-hnpcc-lynch-syndrome./
10. Beitsch PD, Whitworth PW, Hughes K Patel R, Rosen B, Compagnoni G. Underdiagnosis of hereditary breast cancer: Are genetic testing guidelines a tool or an obstacle? J Clin Oncology. 2019;37:453-460.
11. King MC, Levy-Lahad E, Lahad A. Population-based screening for BRCA1 and BRCA2. JAMA. 2014;312:1091-1092.
12. American Society of Breast Surgeons. Consensus guideline on genetic testing for hereditary breast cancer. https://www.breastsurgeons.org/docs/statements/Consensus-Guideline-on-Genetic-Testing-for-Hereditary-Breast-Cancer.pdf
Medical Practice Supplies
VIEW ALL
 Manual Prescription Pad (Large - Yellow)
Manual Prescription Pad (Large - Yellow) Manual Prescription Pad (Large - Pink)
Manual Prescription Pad (Large - Pink) Manual Prescription Pads (Bright Orange)
Manual Prescription Pads (Bright Orange) Manual Prescription Pads (Light Pink)
Manual Prescription Pads (Light Pink) Manual Prescription Pads (Light Yellow)
Manual Prescription Pads (Light Yellow) Manual Prescription Pad (Large - Blue)
Manual Prescription Pad (Large - Blue)__________________________________________________
Appointment Reminder Cards
$44.05
15% Off
$56.30
15% Off
$44.05
15% Off
$44.05
15% Off
$56.30
15% Off
VIEW ALL PRODUCTS
Subscribe to:
Posts (Atom)
















